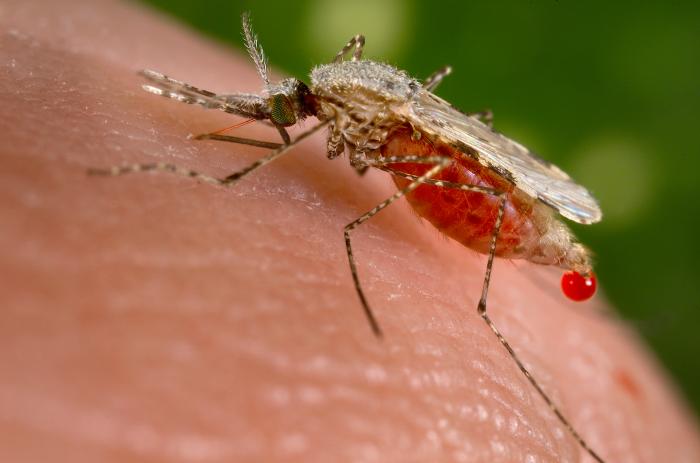
We’ve had to talk a lot about coronavirus vaccines in the past year, both here and elsewhere. But there are other infectious diseases in the world, and other vaccines being developed to prevent their spread. One of the bigger challenges, both in terms of numbers of infections and difficulty in developing a vaccine, is malaria. A variety of malaria vaccine approaches have been proposed and developed. The best result to date was a finding of 55% efficacy in a large scale trial, meaning vaccinated individuals were a little less than half as likely to become infected. Now, a new study of a different vaccine demonstrated 77% efficacy in a moderate scale test, comparable to a phase II trial. While the findings are reported in a preprint with further review pending, and a large scale trial is still forthcoming, this is an encouraging result for a significant public health challenge.
Although numbers have been declining for 15-20 years, there are still roughly 400,000 malaria deaths per year, many in young children. Even if some level of infection persists, reducing the number of deaths would be a meaningful improvement. So that’s the why, but the how of a malaria vaccine is not straightforward. Malaria is caused by a eukaryotic intracellular parasite, not a virus. Viruses have ones to tens of genes packaged in a protein capsule, sometimes with a bit of oily membrane too. in the case of SARS-CoV-2, there is effectively one external protein that antibodies can target, providing a natural focus for vaccine development. By comparison, malaria parasites are entire cells with thousands of genes, multiple external proteins, and several distinct life cycle stages with differences in shape and protein expression. Internally, those cells have a nucleus and mitochondria (with a separate genome) and other organelles, just like our cells. Their life cycle includes a sexual reproductive stage while inside mosquitoes, allowing for the evolutionary leaps of recombination. All of that complexity makes malaria parasites difficult for our immune systems to handle.
What’s more, although we have one name for the disease, there are several distinct species of parasites that cause malaria, including Plasmodium falciparum, Plasmodium knowlesi, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. These are not separated by a few individual point mutations like the headline-making SARS-CoV-2 variants (e.g. B.1.1.7). Their divergence from each other is more comparable to the divergence between gorillas, chimpanzees and bonobos. So immunity to one does not provide immunity to any of the other species. In fact, infection with a single species does not necessarily induce immunity that prevents subsequent infection with that species. More typically, a partial immunity develops which reduces the severity of subsequent infections. This pattern of response is a significant factor in the lethality among small children, since they have not had the prior infections to induce that partial immunity.
Multiple species, numerous evolving antigens, and limited natural immunity present many variables for vaccine development. To simplify, this new vaccine targets a single protein in a single species. The P. falciparum species is especially pathogenic and responsible for the majority of deaths, so that’s where this vaccine is focused. And since some of the P. falciparum proteins show high variability possibly to avoid immune responses, only a more conserved protein is used. Incidentally, since single proteins don’t generate as much of an immune response, the vaccine also includes an adjuvant–basically an immune system activating signal–called Matrix-M, a new product also being used in the Novavax vaccine for SARS-CoV-2. This single-protein approach differs from the previous most successful strategy, which used entire dead parasite cells.
Another advantage of this single-protein approach is that the protein can be grown in yeast cells. This means there’s no requirement for human cell cultures as with some other vaccines. For those with ethical concerns about the origins of certain cell cultures, newer vaccines technologies like these represent a meaningful opportunity to decouple from those cultures. Completely eliminating them from the vaccine pipeline is unlikely to happen all at once, but that doesn’t mean we cannot acknowledge and encourage when significant progress occurs. So really, there are two pieces of good news here: the promise of malaria relief for millions and vaccine manufacturing consistent with a broader range of ethical principles.
Andy has worn many hats in his life. He knows this is a dreadfully clichéd notion, but since it is also literally true he uses it anyway. Among his current metaphorical hats: husband of one wife, father of two teenagers, reader of science fiction and science fact, enthusiast of contemporary symphonic music, and chief science officer. Previous metaphorical hats include: comp bio postdoc, molecular biology grad student, InterVarsity chapter president (that one came with a literal hat), music store clerk, house painter, and mosquito trapper. Among his more unique literal hats: British bobby, captain’s hats (of varying levels of authenticity) of several specific vessels, a deerstalker from 221B Baker St, and a railroad engineer’s cap. His monthly Science in Review is drawn from his weekly Science Corner posts — Wednesdays, 8am (Eastern) on the Emerging Scholars Network Blog. His book Faith across the Multiverse is available from Hendrickson.

Leave a Reply